Free Printable Worksheets Chemistry Using Nernst Equation Pdf Nernst Equation Practice Problems Chemistry Steps General Chemistry Electrochemistry Nernst Equation Practice Problems In the previous post we talked about the Nernst equation which is used to calculate the cell potential under nonstandard conditions based on Eocell and the log of the reaction quotient
The Nernst equation determines the cell potential of reactions that depend on pH If H is involved in the cell reaction the electrochemical potential will depend on the pH Consider the following half reaction 1 H H e The reaction quotient is Q Product Reactant H H 1 2 Nernst Equation Example Problems ECHEM MCD 1 3 02 Estd cell 1 1032 volt Estd cell Ecathode Eanode Eo cell Calculate the standard cell potential for this system Zn s Cu2 1 M Zn 1 M Cu s Reaction The overall electrochemical reaction has the form a Aox b Bred a Ared b Box Ecathode ECu
Free Printable Worksheets Chemistry Using Nernst Equation Pdf
Free Printable Worksheets Chemistry Using Nernst Equation Pdf

Nernst Equation Explained Electrochemistry Example Problems PH

PDF The Nernst Equation From Chemistry To Biology
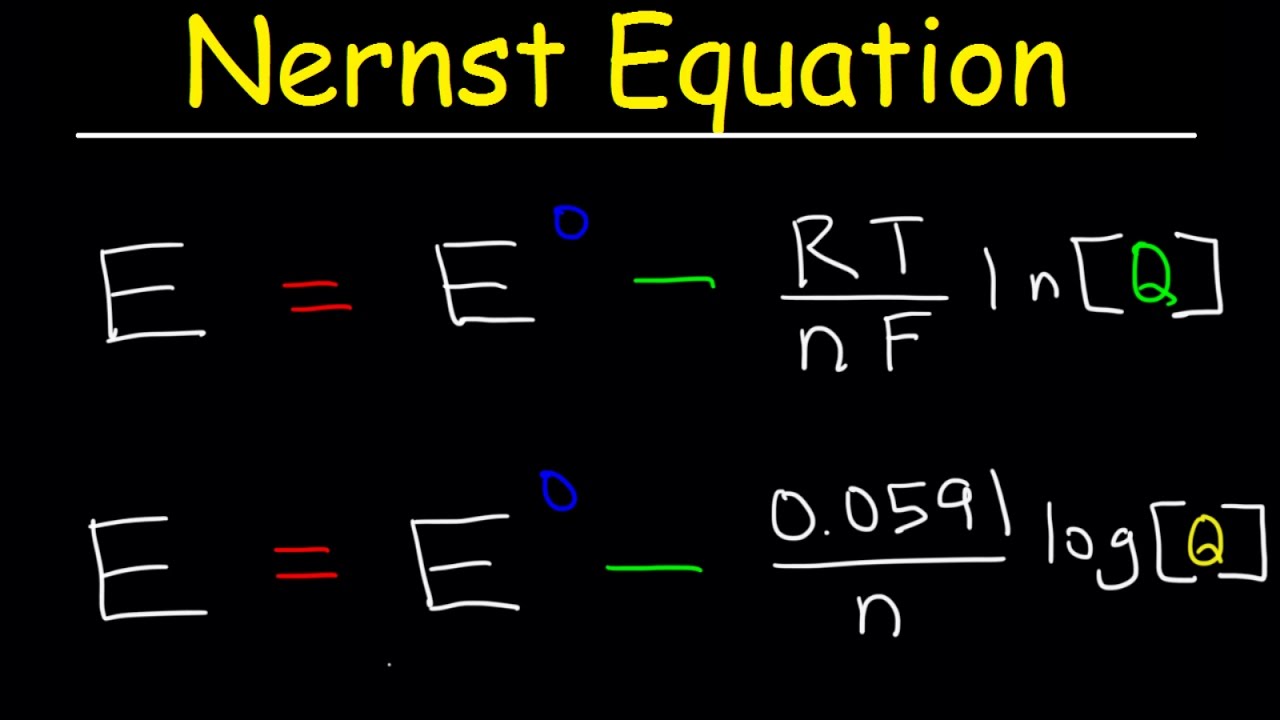
The Nernst Equation Under non standard conditions the cell potential of the half cells is shown by the symbol Ecell The effect of changes in temperature and ion concentration on the Ecell can be deduced using the Nernst equation E electrode potential under nonstandard conditions Using the Nernst equation and the concentrations stated in the problem and n 2 Q Co2 Fe2 0 15M 1 94M 0 077 Now we can insert these into the Nernst Equation at room temperature Equation 17 4 4 Ecell E cell 0 0592 V n logQ 0 17V 0 0592V 2 log0 077 0 17V 0 033V 0 14V
Find the cell potential for the reaction P t 2 M g P t M g 2 at 320 K given the concentration of platinum II ions is four times the concentration of magnesium II ions The value of Relate cell potentials to free energy changes Use the Nernst equation to determine cell potentials at nonstandard conditions Perform calculations that involve converting between cell potentials free energy changes and equilibrium constants
More picture related to Free Printable Worksheets Chemistry Using Nernst Equation Pdf

Important Equations Nonstandard Conditions Nernst Equation Wize

Nernst Equation pdf OneClass
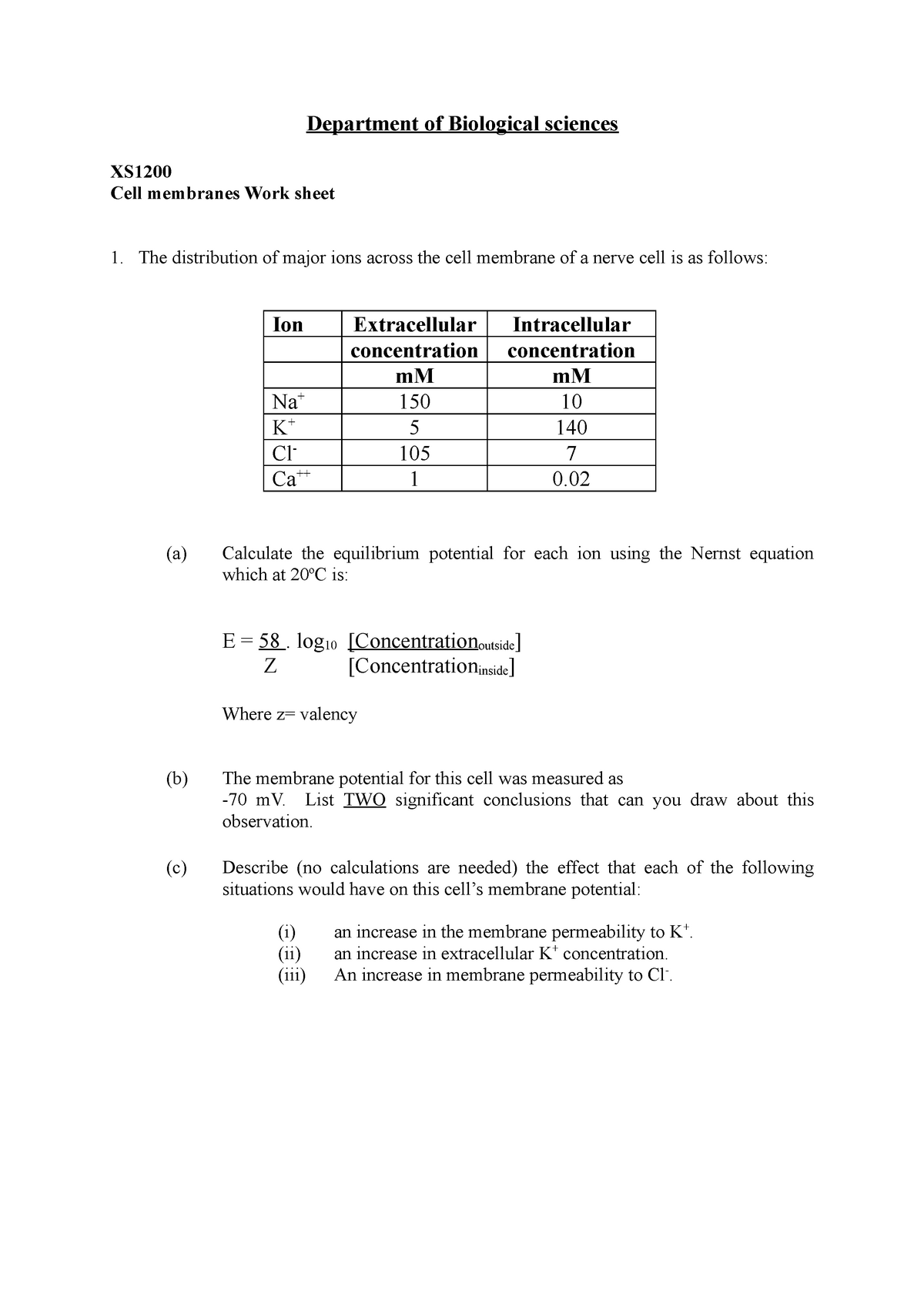
Nernst Equation Worksheet Department Of Biological Sciences XS Cell
Home Bookshelves Analytical Chemistry Supplemental Modules Analytical Chemistry Electrochemistry Basics Nernst Equation Expand collapse global location Nernst Equation Page ID Learning Objectives Explain and distinguish the cell potential and standard cell potential b The Nernst equation for the reduction of Fe 3 is E 77 059 log Q in which Q is the ratio Fe 2 Fe 3 With E set by the O 2 H 2 O couple this becomes 0 82 0 77 0 059 log Q which gives Q 10 0 85 or Fe 2 Fe 3 0 14 1 so the fraction of the iron in its oxidized form is 1 1 14 0 88
19 5 How to Calculate Nonstandard Cell Potential Nernst Equation General Chemistry 11 30 Using the Nernst equation Redox reactions and electrochemistry Chemistry Khan Academy Khan Academy Organic Chemistry Possible Answers Go 120 kJ mol K 4 4 1031 Go 180 kJ mol K 2 8 1031 Go 180 kJ mol K 2 8 10 31 Go 180 kJ mol K 2 8 1031 Go 180 kJ mol K 2 8 10 31 Correct answer Go 180 kJ mol K 2 8 1031 Explanation For this question we re given a redox reaction occurring within a voltaic cell

Nernst Equation Example Problems

15 High School Chemistry Worksheet Answers Worksheeto
https:// general.chemistrysteps.com /nernst-equation...
Nernst Equation Practice Problems Chemistry Steps General Chemistry Electrochemistry Nernst Equation Practice Problems In the previous post we talked about the Nernst equation which is used to calculate the cell potential under nonstandard conditions based on Eocell and the log of the reaction quotient
https://www. chemistrylearner.com /nernst-equation.html
The Nernst equation determines the cell potential of reactions that depend on pH If H is involved in the cell reaction the electrochemical potential will depend on the pH Consider the following half reaction 1 H H e The reaction quotient is Q Product Reactant H H 1 2

Chemistry

Nernst Equation Example Problems
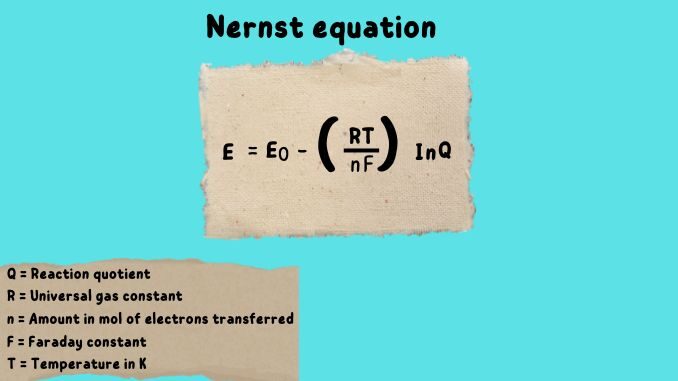
Nernst Equation Definition And Description Science Query

If Ecell E 0cell For Electrochemical Cell Than Which Of The
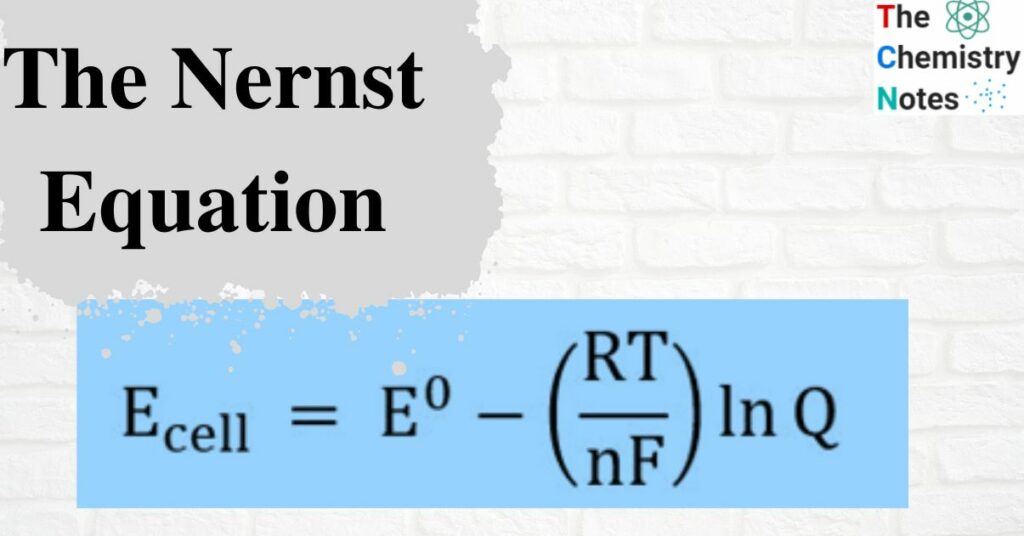
The Nernst Equation Derivation Application And Limitations
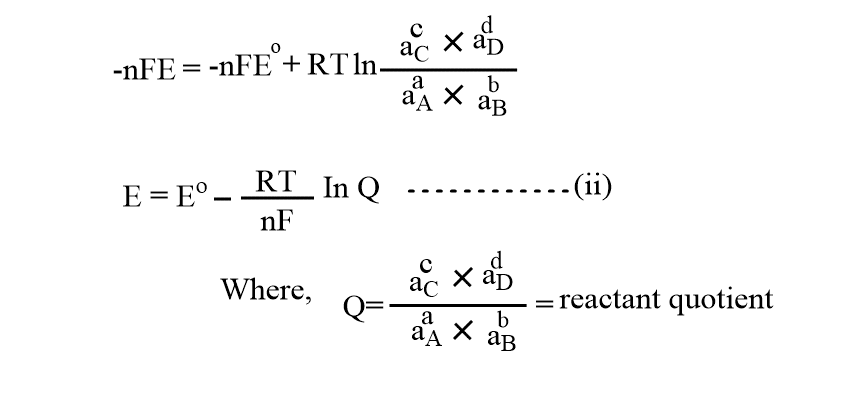
Nernst Equation Definition Derivation Applications Chemistry Notes
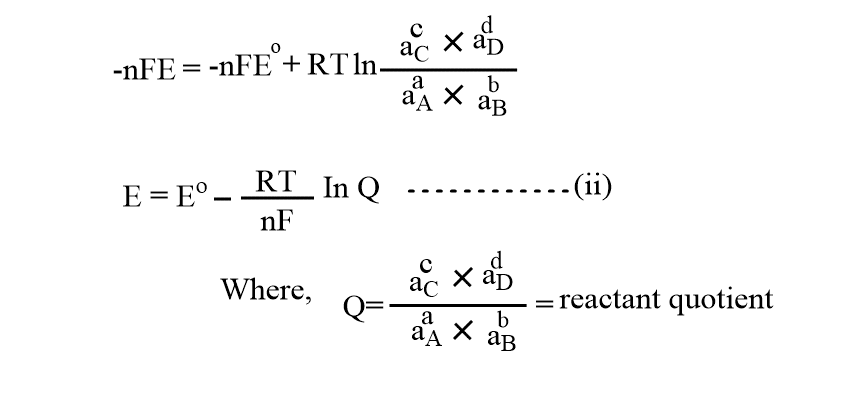
Nernst Equation Definition Derivation Applications Chemistry Notes

Nernst Equation Electrochemistry Simplified Clutch Prep Chemistry

The Worksheet For An Activity To Help Students Learn How To Use
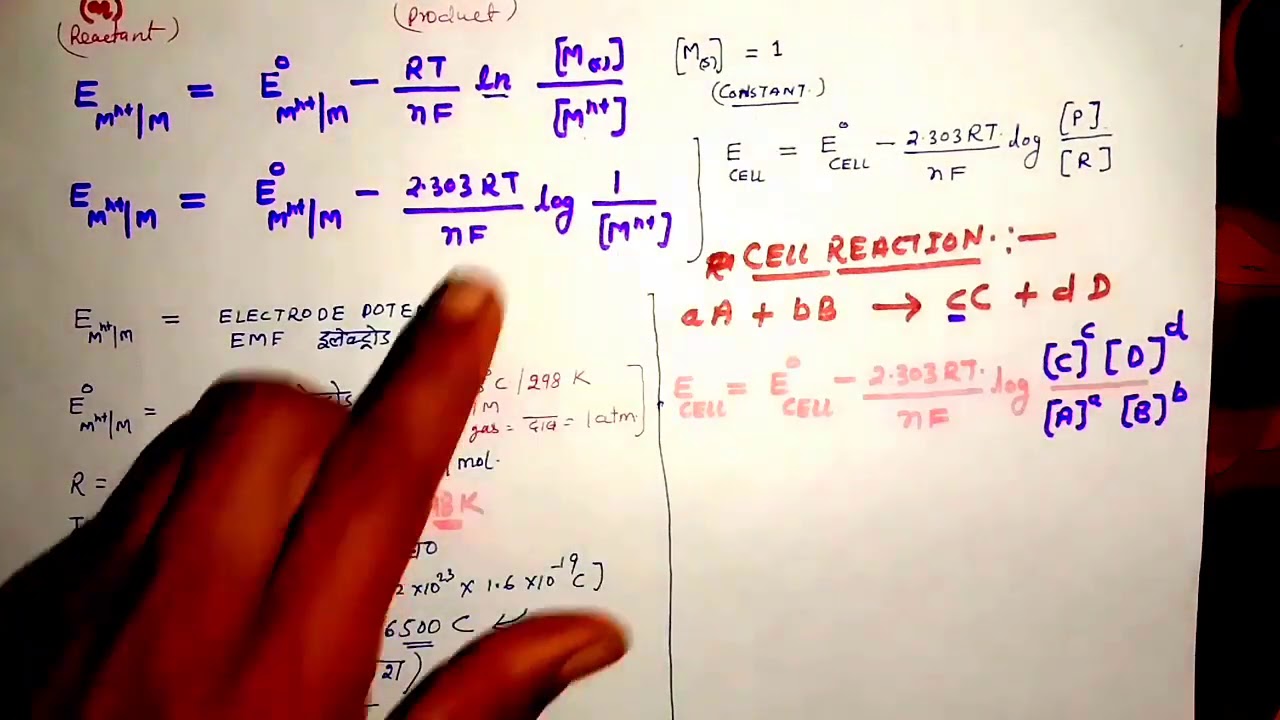
Nernst Equation YouTube
Free Printable Worksheets Chemistry Using Nernst Equation Pdf - Relate cell potentials to free energy changes Use the Nernst equation to determine cell potentials at nonstandard conditions Perform calculations that involve converting between cell potentials free energy changes and equilibrium constants